Chapter 9 - The basics of air preparation
Compressed air
CAUTION! The quality of the compressed air in use is substantially important for the safe operation and durability of the pneumatic system.
Air consists of: Nitrogen (N2) 78,09 %, Oxygen (O2) 20,95% and Argon (Ar) 0,93 %. 0,03 % of the volume is made up by other gases ,such as CO2, Methane and various noble gases. Air can be polluted by sulfurous gases, carbon-monoxides and dirt, damps, or particles.
When producing compressed air (compressing the environmental air), and when transporting it through the tubes, other harmful elements may enter.
In order to define the quality of compressed air, there are standardized purity classes.
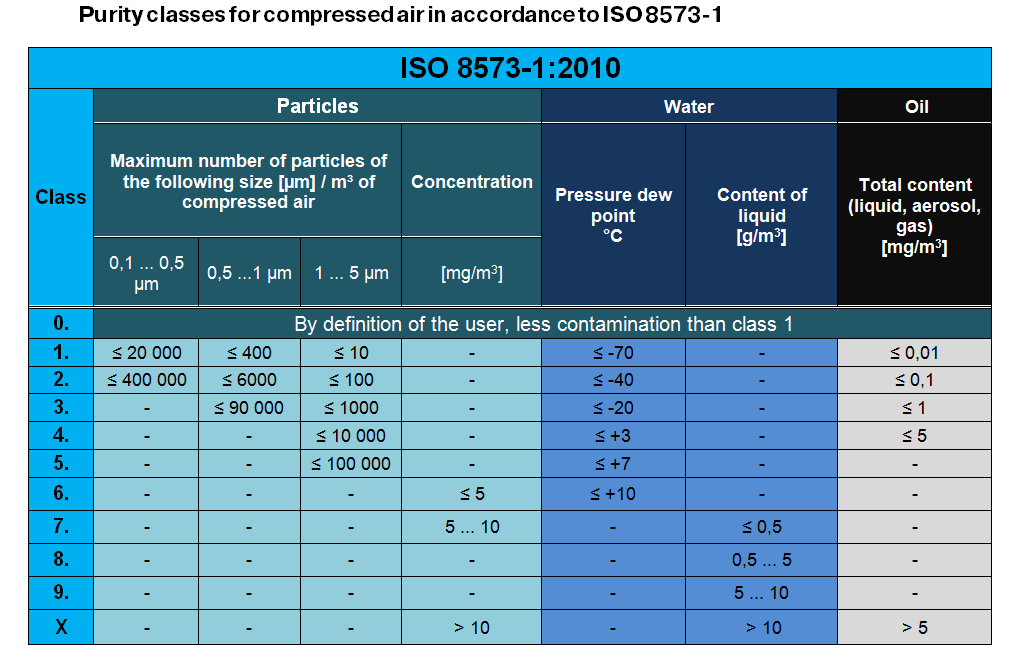
Purity classes for compressed air in accordance to ISO 8573-1
Compressed air is classified. The three most important elements of pollution are particles, water and oil.
They are classified in accordance to their degree of concentration within the air and displayed as follows:
ISO 8573-1:2010 [A:B:C]
- A – particles | 0 ... 8, X
- B – water | 0 ... 9, X
- C – oil | 0 ... 4, X
In case an element is displayed as class X (= an element with a high concentration), its amount or the degree of concentration needs to be put in round brackets. The following example shows the quality of air where the water concentration is at Cw 15 g/m3. Therefore we indicate its purity as follows:
ISO 8573-1:2010 [4:X(15):3]
In normal pneumatic applications the following air quality is sufficient: ISO 8573-1:2010 [7:4:4].
According to the ISO norm, the permitted degrees of pollution are:
- Particle concentration 5-10 mg/m3
- Dew point less than 3 °C
- Oil concentration max. 5 mg/m3
For specific applications or in extreme environments (e.g. railway application in cold climates), a higher air purity might be required.
Basics regarding the generation and preparation of compressed air
When generating compressed air, it is important to ensure it to be as oil-free as possible at the lowest possible cost. The preparation of compressed air has the same economical aspect.
It is possible to generate compressed air of a high quality – i.e. oil-free or with reduced oil-content – with compressors that work both with and without lubrication if a sound air preparation has been established.
The environmental air and its quality
The air's quality highly depends on external, environmental factors. The concentration of hydro-carbonates due to industry or traffic can reach levels of 4-14 mg/m3.
In factories the oil content can exceed 10 mg/m3 because of coolant and lubrication fluids in the machinery.
Moreover there are other polluting elements such as sulfur dioxide, soot, metals, dust and humidity.
How to define „oil-free“ compressed air?
According to ISO 8573-1 compressed air can be called “oil-free“ when the content of oil (including oil dust) is less than 0,01 mg/m3. That is about 4 % of the oil content in normal air, so it is hardly detectable. You can find such requirements for very high air purity e.g. in the food and pharmaceutical industry as well as the electronics industry (manufacture of wafers etc.).
Where does humidity come from?
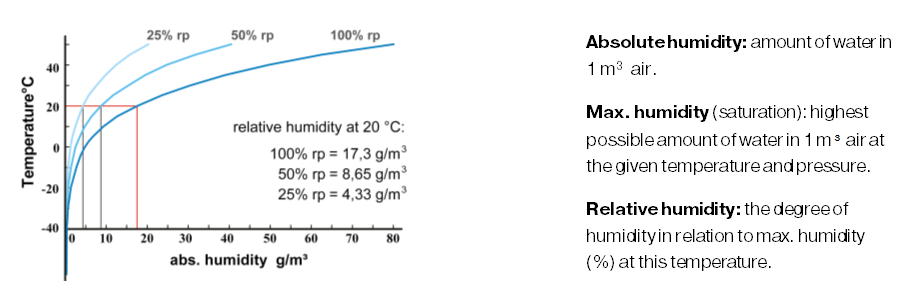
There is always humidity in the environmental air. The degree of the humidity depends on temperature and air-pressure. The warmer it is, the higher the ability of air to hold water. At higher pressure that ability weakens.
If the humidity becomes higher than 100% relative humidity the excess water is released. The temperature at which water is released at the given air-water concentration is called the dew point.
Water is released when the temperature goes down and/or when air pressure rises. This is exactly what happens in the compressor as well as in the air-cooler. The water that is released forms the so-called condensate.
The drying of compressed air
When air gets cooler, it releases water.
Example:
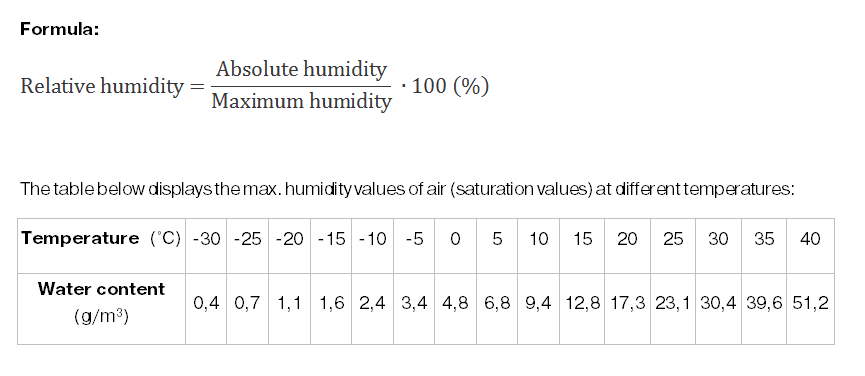
A screw compressor working at a temperature of 20°C, at sea-level, has a suction capacity of 10 m3 per minute; the relative humidity of the environmental air is 60 %.
Looking at the table above, we can see that at 20°C 100% humidity = 17.3 g water / m3 air. So we can deduct that 60% humidity = 10.4 g water / m3
Thus the 10 m³ contains 104 g of water.
At a compression ratio of 1:10 (10 bar), the compressor generates 1 m³ of compressed air per minute (10 m3 environmental air = 1 m3 compressed air).
During compression the air's temperature rises to around 80 °C. At this temperature it can hold 290 g water / m3 air (referring again to the table above). Therefore its relative humidity is at only 36% (104 / 290 = 36%). The air is relatively dry and produces no condensate.
A cooler after the compressor cools the compressed air down from 80 °C to approx. 35 °C. At 35°C the air can only hold 39.6 g / m3 , though there are 104 g of water inside each m3. So 64 g / m3 will be released. This means we have 64 g of excess water every minute the compressor operates. This translates (x 60 x 8) into 31 liter of condensate after an 8 hour shift.
In order to have fairly safe working conditions, this condensate needs to be removed. The drying ( = cooling) of the generated compressed air is an essential part of the generation and preparation of compressed air.
CAUTION! Without properly drying the air you will find a lot of condensate in the air-tank as well as in your pipe-lines, machinery and many other places.
CAUTION! The standard filters of an FRL-unit (50 ... 0,01 Mikron), do not influence the content of water. They are made for filtering particles. The water you will find in the condensate drain of a filter consists only of a few drops. It is irrelevant in comparison to the amounts of water mentioned before.
How to dry the air?
The drying of compressed air in an industrial environment is usually achieved with one of the following methods:
- Deliquescent dryer
A deliquescent dryer typically consists of a container filled with hygroscopic material that absorbs the water. Advantage: No additional energy is required. Disadvantage: The hygroscopic materials have to be replaced regularly. - Desiccant dryer
Also called twin tower dryer or adsorption dryer. The air flows through a desiccant material such as silica gel. The gel's ability to keep water is limited, but can be easily reset by blowing the water out (“purging” the gel). No additional energy is required here either, but there is a loss of compressed air due to the purge. Large equipment is needed for air flows at high speeds. - Membrane dryer
First the air has to be filtered with a high quality coalescing filter, then the air passes through a center bore of a hollow fibre in a membrane bundle. Dryer air is floating outside the membrane. This leads to an exchange of vapor. Disadvantage: the flow is limited to around 1000 l/min. - Refrigerated dryer
Refrigerated drying is based on the principle that colder air can hold less water. The air passes a heat-exchanger that is cooled to around 3°C. The cooled-down air loses water as well as oil, both of which are collected. After drying the air is filtered.
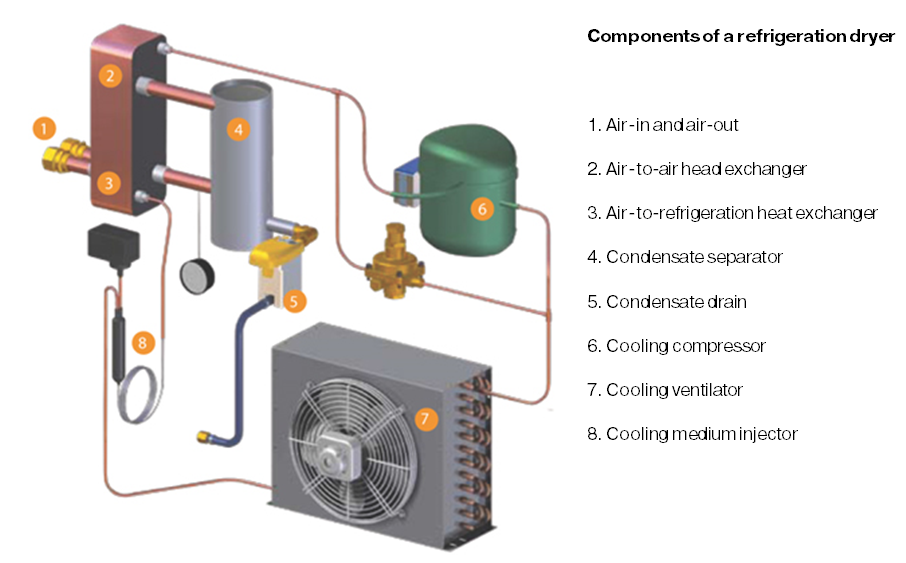
Why is air-preparation necessary?
You could look at a compressor as a big vacuum cleaner. It sucks in everything from the environment. When generating compressed air, all the environmental elements of pollution are concentrated. Everything is fed into the pneumatic system.
FRL-units are an important element of the pneumatic system. With these units the air can reach the required quality since they have filters for cleanness, lubrication for oil content and can manage pressure. Well-processed air does not only ensure a safer workspace but also increases the durability of the equipment.
Air preparation equipment consists of:
- Filters
- Pressure regulators
- Lubricators
- Switch-on and starter valves
- Distributors, pressure switches
We can categorize them by design, size, flow rates and port size. There is a wide range available, from port size G1/8" to G3".
The following pictures show a selection of the most common elements: